We have applied the QCPI methodology to study the dynamics of the ferrocene-ferrocenium charge transfer reaction in liquid hexane with unprecedented accuracy. The charge transfer complex was surrounded by 66 hexane molecules (1,320 atoms), interacting via CHARMM force fields with periodic boundary conditions.
In this case of a fast electron transfer reaction, the donor population was seen to exhibit non-exponential kinetics. It is evident that the long-time rate constant is not representative of the time for completion of this reaction.
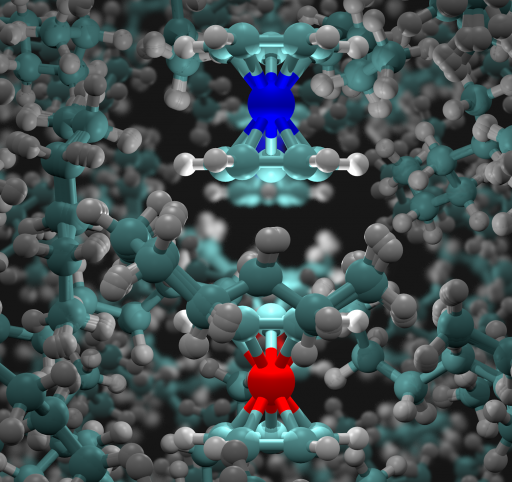
The QCPI methodology captures faithfully the solute-solvent interaction. Through this interaction, the quantum mechanical delocalization of the system spreads to the classical solvent. The figure a snapshot of the simulation and the superposition of quantum-classical paths, whose phases are fully accounted for in the QCPI simulation. A video is also available.
Related Articles: